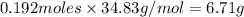Answers: 1
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 01:00, only1cache
Which is true concerning the products and reactants of photosynthesis and cellular respiration? a. the products of photosynthesis are sugars and the reactants of cellular respiration are starches. b. the products of photosynthesis are reactants in cellular respiration. c. oxygen is needed for photosynthesis and is given off in cellular respiration.
Answers: 2


Chemistry, 23.06.2019 09:20, taylorannsalazar
La reaccion entre monoxido de nitrogeno (no) y oxigeno para formardioxido de nitrogeno (no2) es un paso determinante para la formacion del smog, la reaccion es la siguiente: 2no + o2 = 2no2 cual sera el numero de moles de no2 que se formaran por la reaccion completa de 8 moles de oxigeno con suficiente monoxido?
Answers: 1
Do you know the correct answer?
Lithium and nitrogen react in a combination reaction to produce lithium nitride: 6li (s) + n2 (g) →...
Questions in other subjects:


Mathematics, 28.06.2019 17:30

Social Studies, 28.06.2019 17:30



Business, 28.06.2019 17:30





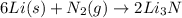
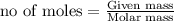
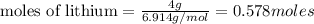
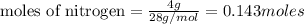
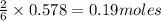 of lithium nitride.
of lithium nitride.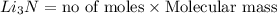
 =
=