
Chemistry, 28.06.2019 02:30, Austin4094
Potassium hydroxide (koh) reacts with sulfuric acid (h2so4) to produce potassium sulfate (k2so4) and water. what best describes this reaction? a single replacement reaction occurs because potassium ions bond with sulfate ions to form a salt. a double replacement reaction occurs because potassium ions bond with sulfate ions to form a salt. a single replacement reaction occurs because potassium is more reactive than hydrogen. a double replacement reaction occurs because potassium is less reactive than hydrogen.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:30, khalaflaf2684
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 07:00, erickamurillo9929
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 22.06.2019 08:30, MacenParisi
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2
Do you know the correct answer?
Potassium hydroxide (koh) reacts with sulfuric acid (h2so4) to produce potassium sulfate (k2so4) and...
Questions in other subjects:

Mathematics, 15.12.2021 01:50



Mathematics, 15.12.2021 01:50



Mathematics, 15.12.2021 01:50

History, 15.12.2021 01:50




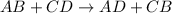
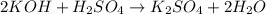
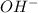 ion of KOH combines with
ion of KOH combines with 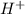 ion of sulfuric acid to give water.
ion of sulfuric acid to give water.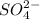 ) of sulfuric acid gets combine with two potassium ion (
) of sulfuric acid gets combine with two potassium ion (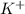 )to give salt of potassium sulfate,
)to give salt of potassium sulfate, 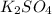 .
.




