
Chemistry, 28.06.2019 02:30, jobucks9976
Fe3+(aq) (yellow) + scn-(aq) (colorless) fescn2+(aq) (blood-red) chloride ions are colorless. potassium ions are also colorless. the above equilibrium can be created by mixing an iron (iii) chloride solution with a potassium thiocyanate solution. based on this information and the colors in the equilibrium above answer the first two questions. 1. what color would an fecl3 solution be? 2. what color would a kscn solution be? 3. what color do you get when you mix fecl3 and kscn as shown in the video for the control test tube?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:10, cordovamaria22
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1

Chemistry, 22.06.2019 10:00, JOEFRESH10
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 18:00, jalenclarke25
What volume would 2.25 moles of ne has occupy at stp?
Answers: 1

Chemistry, 22.06.2019 19:50, jakaylathomas11
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3
Do you know the correct answer?
Fe3+(aq) (yellow) + scn-(aq) (colorless) fescn2+(aq) (blood-red) chloride ions are colorless. potass...
Questions in other subjects:

Physics, 25.05.2021 04:40

Mathematics, 25.05.2021 04:40

Mathematics, 25.05.2021 04:40

History, 25.05.2021 04:40

Mathematics, 25.05.2021 04:40



Mathematics, 25.05.2021 04:40

English, 25.05.2021 04:40


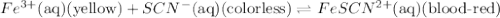
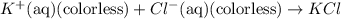
![FeCl_3+K(SCN)\rightleftharpoons KCl+[Fe(SCN)]Cl_2(blood-red)](/tpl/images/0025/5430/e3b23.png)
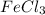 we will get blood red color solution of
we will get blood red color solution of ![[FeSCN]Cl_2](/tpl/images/0025/5430/ecffe.png) .
.




