
Chemistry, 28.06.2019 03:00, jetblackcap
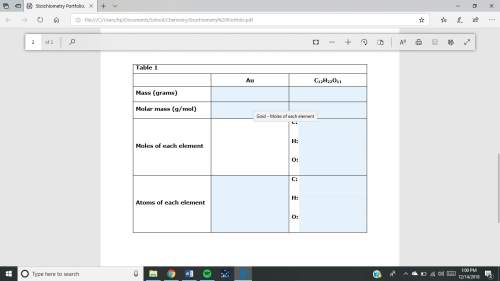
1. a sample of gold (au) has a mass of 35.12 g. a. calculate the number of moles of gold (au) in the sample and record in table 1. show your work. b. calculate the number of atoms of gold (au) in the sample and record in table 1. show your work. 2. a sample of table sugar (sucrose, c12h22o11) has a mass of 1.202 g. a. calculate the number of moles of c12h22o11 contained in the sample and record in table 1. show your work. b. calculate the moles of each element in c12h22o11 and record in table 1. show your work. c. calculate the number of atoms of each type in c12h22o11 and record in table 1. show your work. table looks like this: column 1 column 2 column 3 au c12h22o11mass (grams)molar mass (g/mol)moles of each element c: h: o: atoms of each element c: h: o: fast!

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:40, eamccoy1
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2

Chemistry, 22.06.2019 13:30, bryce99
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 19:10, krisandlance
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 22.06.2019 19:50, nikoidurrant
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2
Do you know the correct answer?
1. a sample of gold (au) has a mass of 35.12 g. a. calculate the number of moles of gold (au) in the...
Questions in other subjects:

English, 05.10.2020 15:01


Health, 05.10.2020 15:01


Mathematics, 05.10.2020 15:01

English, 05.10.2020 15:01


World Languages, 05.10.2020 15:01







