
Chemistry, 28.06.2019 09:00, Zachary4759
The enthalpy change for converting 1.00 mol of ice at -25.0 ∘c to water at 90.0∘c is kj. the specific heats of ice, water, and steam are 2.09 j/g−k, 4.18 j/g−k, and 1.84 j/g−k, respectively. for h2o, δ hfus = 6.01kj/mol, and δhvap = 40.67 kj/mol.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, hdjsjfjruejchhehd
Define dew point. i am writing this part to be able to ask the question
Answers: 1


Chemistry, 22.06.2019 22:30, kristen17diaz
How many valence electrons are in atom of radon?
Answers: 1

Chemistry, 22.06.2019 23:00, Kykebailey2356
Which of two curves exhibits exponential growth
Answers: 1
Do you know the correct answer?
The enthalpy change for converting 1.00 mol of ice at -25.0 ∘c to water at 90.0∘c is kj. the specif...
Questions in other subjects:

Geography, 19.10.2019 03:40

Arts, 19.10.2019 03:40





Mathematics, 19.10.2019 03:40

Computers and Technology, 19.10.2019 03:40

Mathematics, 19.10.2019 03:40

Mathematics, 19.10.2019 03:50


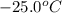 to water at
to water at 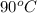 is, 7.712 KJ
is, 7.712 KJ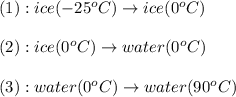
![\Delta H=[m\times c_{ice}\times (T_2-T_1)]+\Delta H_{fusion}+[m\times c_{water}\times (T_3-T_2)]](/tpl/images/0026/6160/eeaad.png)
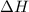 = enthalpy change
= enthalpy change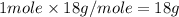
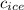 = specific heat of ice = 2.09 J/gk
= specific heat of ice = 2.09 J/gk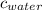 = specific heat of water = 4.18 J/gk
= specific heat of water = 4.18 J/gk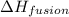 = enthalpy change for fusion = 6.01 KJ/mole = 0.00601 J/mole
= enthalpy change for fusion = 6.01 KJ/mole = 0.00601 J/mole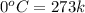
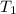 = initial temperature of ice =
= initial temperature of ice = 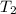 = final temperature of ice =
= final temperature of ice = 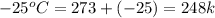
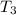 = initial temperature of water =
= initial temperature of water = 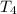 = final temperature of water =
= final temperature of water = 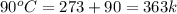
![\Delta H=[18g\times 2.09J/gK\times (273-248)k]+0.00601J+[18g\times 4.18J/gK\times (363-273)k]](/tpl/images/0026/6160/21744.png)
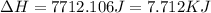 (1 KJ = 1000 J)
(1 KJ = 1000 J)



