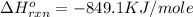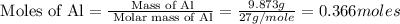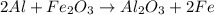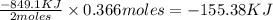Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:30, ansuaprajita1506
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 14:20, greenbyron88
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 21:40, fatherbamboo
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3
Do you know the correct answer?
Determine the heat given off to the surroundings when 9.873 g of aluminum reacts according to the eq...
Questions in other subjects:




Mathematics, 03.12.2021 20:00

Mathematics, 03.12.2021 20:00


Chemistry, 03.12.2021 20:00


Mathematics, 03.12.2021 20:00