Chemistry, 28.06.2019 14:00, spiderman66
Solid sodium reacts violently with water, producing heat, hydrogen gas, and sodium hydroxide. how many molecules of hydrogen gas are formed when 48.7 g of sodium are added to water? show your work. (4 points) 2na + 2h2o > 2naoh + h2 2na + 2h2o > 2naoh + h2 48.7 g ? molecules 2 mol na= 1 mol h2 1 mol na= 23g na 1 mol h2= 6.02x1023 molecules (48.7g na/1)x(1 mol na/23g na)x(1 mol h2/2mol na)x(6.02x1023/1 mol h2) (48.7)(6.02x1023)/(23)(2)= 293.174x1023 molecules can someone tell me what i did wrong?

Answers: 1
Similar questions



Do you know the correct answer?
Solid sodium reacts violently with water, producing heat, hydrogen gas, and sodium hydroxide. how ma...
Questions in other subjects:

Health, 01.09.2020 01:01

Mathematics, 01.09.2020 01:01


History, 01.09.2020 01:01


Mathematics, 01.09.2020 01:01

Mathematics, 01.09.2020 01:01



Biology, 01.09.2020 01:01


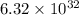
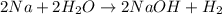
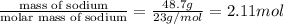
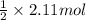 of hydrogen gas that is 1.05 moles of hydrogen gas.
of hydrogen gas that is 1.05 moles of hydrogen gas.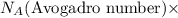 moles of substance
moles of substance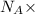 moles of hydrogen gas
moles of hydrogen gas