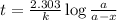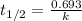Chemistry, 28.06.2019 14:00, lwilliams28
Which statements correctly describe the decay rates of radioactive isotopes? a} it takes two half-lives for a sample to fully decay. b} the exact time when an individual atom will decay can be accurately predicted. c} after each half-life, the amount of radioactive material is reduced by half. d) all radioactive isotopes have the same half-life. e} the decay of individual atoms in a sample of radioactive material is random.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 04:40, yayamcneal05
[01.07]what is the answer to the problem: 101 g + 25.01 g + 5.05 g? 131.06 g 131.1 g 131 g 130 g
Answers: 1


Chemistry, 23.06.2019 18:10, CarlosCooke2
How many moles of water are produced if 5.43 mil pb02 are consumed?
Answers: 3
Do you know the correct answer?
Which statements correctly describe the decay rates of radioactive isotopes? a} it takes two half-...
Questions in other subjects:


History, 10.02.2021 17:20

Mathematics, 10.02.2021 17:20

Mathematics, 10.02.2021 17:20


Geography, 10.02.2021 17:20

History, 10.02.2021 17:20


Mathematics, 10.02.2021 17:20

Mathematics, 10.02.2021 17:20