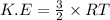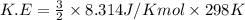Chemistry, 28.06.2019 14:30, dsdlskds3880
Calculate the kinetic energy, in j/mole, of 1.00 mole of gaseous water molecules at room temperature (25.0ºc).

Answers: 1
Other questions on the subject: Chemistry




Chemistry, 23.06.2019 10:30, malum2009
Can anyone explain 1. review your spectrometry data and use the known elements to identify the star's composition. which unknown elements make up this star? justify your element selections. 2. in parts i and ii of the lab, what happened to the electrons of each element to produce the different colors of light? explain your answers using important terms from the lesson and information provided in the laboratory. 3. stars composed of heavier (more massive) elements are often slightly older than stars made predominantly from hydrogen and helium. based on your data, is the newly discovered star a younger star? explain your answer.
Answers: 2
Do you know the correct answer?
Calculate the kinetic energy, in j/mole, of 1.00 mole of gaseous water molecules at room temperature...
Questions in other subjects:

Chemistry, 09.03.2021 21:40



Biology, 09.03.2021 21:40

Mathematics, 09.03.2021 21:40

English, 09.03.2021 21:40

Mathematics, 09.03.2021 21:40

English, 09.03.2021 21:40

Mathematics, 09.03.2021 21:40

Mathematics, 09.03.2021 21:40