
Chemistry, 28.06.2019 17:30, Itsyourgirllulu
You wish to measure the iron content of the well water on the new property you are about to buy. you prepare a reference standard fe3 solution with a concentration of 5.17 ă— 10-4 m. you treat 13.0 ml of this reference with hno3 and excess kscn to form a red complex, and dilute the reference to 45.0 ml. the diluted reference is placed in a cell with a 1.00-cm light path. you then take 30.0 ml of the well water, treat with hno3 and excess kscn, and dilute to 100.0 ml. this diluted sample is placed in a variable pathlength cell. the absorbance of the reference and the sample solutions match when the pathlength is 4.78 cm. what is the concentration of iron in the well water? for each solution, the zero is set with a blank.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 23:40, sydneykated
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2

Chemistry, 23.06.2019 00:00, PlzNoToxicBan
What is the pressure of 0.500 moles of carbon dioxide gas in a 2.5 l tank and at a temperature of 301 k? (r=0.0821 l·atm/mol·k) 3.08 atm 1.2 atm 0.23 atm 4.01 atm 4.94 atm
Answers: 1

Chemistry, 23.06.2019 02:30, kieante01
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1

Chemistry, 23.06.2019 06:40, Science2019
How many joules of heat are required to raise thetemperature of 750 g of water from 11.0 °c to 19.0 °c?
Answers: 1
Do you know the correct answer?
You wish to measure the iron content of the well water on the new property you are about to buy. you...
Questions in other subjects:



Chemistry, 11.03.2020 23:24








 M
M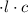 by the Beer-Lambert law, where
by the Beer-Lambert law, where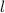 the path length, ε the molar absorptivity of the solute, and
the path length, ε the molar absorptivity of the solute, and concentration of the solution.
concentration of the solution.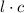 is constant;
is constant; 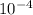 M. Diluting it to 45.0 mL results in a concentration of
M. Diluting it to 45.0 mL results in a concentration of 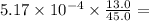 1.494 M.
1.494 M.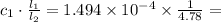 3.126 M.
3.126 M.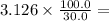 1.04 ⨯
1.04 ⨯ 





