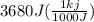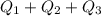Chemistry, 28.06.2019 18:00, nuconteaza119
Ethanol (c2h5oh) melts at -114â°c. the enthalpy of fusion is 5.02 kj/mol. the specific heats of solid and liquid ethanol are 0.97 j/gk and 2.3 j/gk, respectively. how much heat (kj) is needed to convert 25.0 g of solid ethanol at -135â°c to liquid ethanol at -50â°c?

Answers: 1
Similar questions

Chemistry, 28.06.2019 18:00, itscheesycheedar
Answers: 1

Chemistry, 28.06.2019 18:00, diazsindy
Answers: 1

Chemistry, 23.07.2019 06:00, daijahbuck
Answers: 1

Chemistry, 31.07.2019 15:10, tamiawilliams3pe55hs
Answers: 1
Do you know the correct answer?
Ethanol (c2h5oh) melts at -114â°c. the enthalpy of fusion is 5.02 kj/mol. the specific heats of soli...
Questions in other subjects:

English, 29.05.2020 04:01

Business, 29.05.2020 04:01

Mathematics, 29.05.2020 04:01


Mathematics, 29.05.2020 04:01

Social Studies, 29.05.2020 04:01


Social Studies, 29.05.2020 04:01

Mathematics, 29.05.2020 04:01

Mathematics, 29.05.2020 04:01


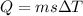
 is the change in temperature.
is the change in temperature.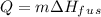
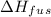 is the enthalpy of fusion.
is the enthalpy of fusion.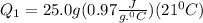
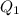 = 509.25 J
= 509.25 J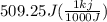
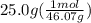
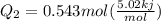
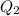 = 2.72 kj
= 2.72 kj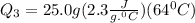
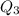 = 3680 J
= 3680 J