
Mrs. smith is demonstrating a chemical change for her class. she places 15 grams of baking soda into a beaker. next she adds 15 grams of vinegar to the same beaker. when the two compounds make contact, they bubble and fizz a great deal. she places the beaker on the balance and notes that the mass of the solution in the beaker is less than the expected 30 grams. why is the mass of the solution in the beaker less than 30 grams? a) the balance was not working correctly. b) the gas that was released changed the mass. c) mass is always lost in a chemical reaction. d) the new products have less mass than the original reactants.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:50, justabeachbum
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 04:20, lex68259100
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 10:00, berniceallonce22
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1
Do you know the correct answer?
Mrs. smith is demonstrating a chemical change for her class. she places 15 grams of baking soda into...
Questions in other subjects:








English, 24.06.2019 03:30

English, 24.06.2019 03:30

Mathematics, 24.06.2019 03:30


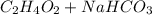 →
→ 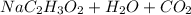 ↑
↑



