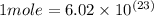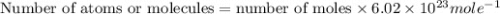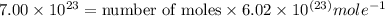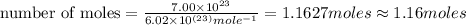Chemistry, 28.06.2019 20:30, truthqmatic16
In the world of chemistry, a 'mole' is a number of a very large number of something. just as a 'dozen' is 12 of something, a 'mole' is about 6.02 * 10^23 of something. in chemistry, we like to measure the number of atoms or molecules in moles. if one mole is equal to 6.02 * 10^23 atoms, and you have 7.00 * 10^23 atoms, then how many moles do you have? round your answer to two decimal places.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, sethjohnson386pbnm3x
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 08:20, pilarmonsivais
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3

Chemistry, 22.06.2019 11:50, trinityrae4657
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 14:00, BrandyLeach01
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3
Do you know the correct answer?
In the world of chemistry, a 'mole' is a number of a very large number of something. just as a 'do...
Questions in other subjects:

Chemistry, 13.05.2021 19:50

Mathematics, 13.05.2021 19:50

Mathematics, 13.05.2021 19:50


Mathematics, 13.05.2021 19:50

Mathematics, 13.05.2021 19:50


Spanish, 13.05.2021 19:50



 numbers of atoms.
numbers of atoms.