Chemistry, 28.06.2019 22:00, sarbjit879
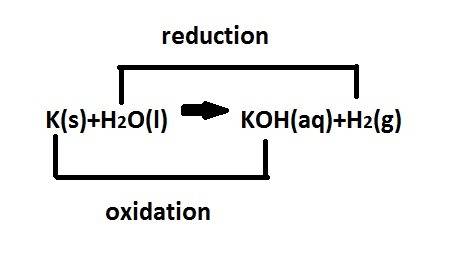
When potassium metal is placed in water a large amount of energy is released as potassium hydroxide and hydrogen gas are produced in the reaction 2k(s)+2h2o=2koh(aq)+h2(g). your lab partner says this is a redox reaction and a combustion reaction. do you agree? defend your answer by explaining whether or not it meets the requirements of each type of reaction.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:00, davisnaziyahovz5sk
The arrangement of particles is most ordered in a sample of
Answers: 1

Chemistry, 22.06.2019 20:30, sydneip6174
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2

Chemistry, 22.06.2019 22:30, kristen17diaz
How many valence electrons are in atom of radon?
Answers: 1
Do you know the correct answer?
When potassium metal is placed in water a large amount of energy is released as potassium hydroxide...
Questions in other subjects:

Physics, 17.01.2021 01:00



English, 17.01.2021 01:00

English, 17.01.2021 01:00

Mathematics, 17.01.2021 01:00





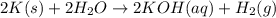
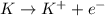 oxidation
oxidation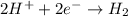 reduction
reduction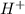 ions of water by gaining electrons (from potassium in water) gives hydrogen gas.
ions of water by gaining electrons (from potassium in water) gives hydrogen gas.