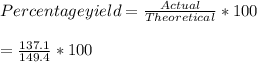Chemistry, 29.06.2019 02:00, 101EXPERIENCE
Consider the following reaction: 2h2s (g) + 3o2 (g) 2so2 (g) + 2h2o (g) if o2 was the excess reagent, 8.3 mol of h2s were consumed, and 137.1 g of water were collected after the reaction has gone to completion, what is the percent yield of the reaction? show your work.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, freddhendrickss
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 11:00, Usman458
The twister and runaway train are two coasters at the same amusement park. both coasters start at the same height. the coaster for the twister is twice the mass of the coaster for the runaway train. which roller coaster has greater gravitational potential energy at the start of the ride?
Answers: 1

Chemistry, 22.06.2019 16:00, rorymartin04
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2
Do you know the correct answer?
Consider the following reaction: 2h2s (g) + 3o2 (g) 2so2 (g) + 2h2o (g) if o2 was the excess reag...
Questions in other subjects:

History, 17.10.2019 14:00

History, 17.10.2019 14:00


English, 17.10.2019 14:00

History, 17.10.2019 14:00





English, 17.10.2019 14:10