Chemistry, 29.06.2019 02:30, joshuakirby
Calculate how many grams of sodium azide (nan3) are needed to inflate a 25.0 × 25.0 × 20.0 cm bag to a pressure of 1.35 atm at a temperature of 20.0°c. how much sodium azide is needed if the air bag must produce the same pressure at 10.0°c? the equation is 20nan3+6sio2+4kno3=32n2+5na4sio4+k4 sio

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, adrian08022
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2


Chemistry, 22.06.2019 12:00, winterblanco
What is the lowest number energy level where a d sublevel is found
Answers: 1

Chemistry, 22.06.2019 15:30, 20cschultz
Which suspect most likely committed the robbery and how do you know
Answers: 2
Do you know the correct answer?
Calculate how many grams of sodium azide (nan3) are needed to inflate a 25.0 × 25.0 × 20.0 cm bag to...
Questions in other subjects:

History, 16.12.2019 01:31


History, 16.12.2019 01:31



Mathematics, 16.12.2019 01:31





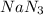 at temperature
at temperature 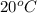 28.47 g.
28.47 g. 29.51 g.
29.51 g.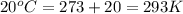


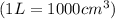
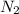 .
.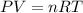
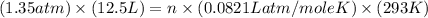


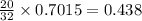 moles of
moles of 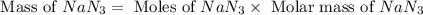
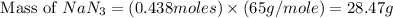
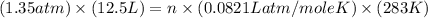
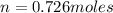
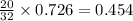 moles of
moles of