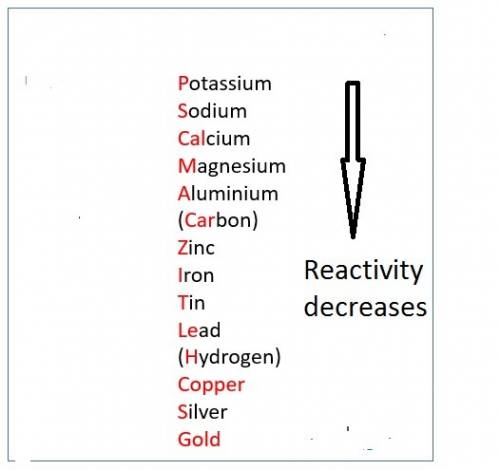
When hno3 and au are mixed together, what do you expect to happen, and why? a. no reaction, because au is above h on the activity series and they cannot react b. no reaction, because au is lower on the activity series and cannot replace h c. they form au(no3)2 and h2, because au is more reactive and able to replace h d. they form au(no3)2 and h2, because they trade places in a double replacement reaction

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:30, kkelley9223
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 09:30, junkmailemail42
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 22.06.2019 10:30, ashlpiriz123
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1
Do you know the correct answer?
When hno3 and au are mixed together, what do you expect to happen, and why? a. no reaction, because...
Questions in other subjects:

Mathematics, 28.10.2020 20:00

Biology, 28.10.2020 20:00

Arts, 28.10.2020 20:00


History, 28.10.2020 20:00

Mathematics, 28.10.2020 20:00


History, 28.10.2020 20:00

Mathematics, 28.10.2020 20:00

Mathematics, 28.10.2020 20:00


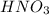 . Thus there will be no reaction.
. Thus there will be no reaction.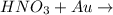 no reaction
no reaction