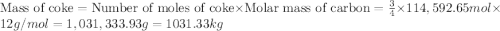The reduction of iron(iii) oxide (fe2o3) to pure iron during the first step of steelmaking, 2fe2o3(s)→ 4fe(s)+ 3o2(g) is driven by the high-temperature combustion of coke, a purified form of coal: c(s)+ o2(g)→ co2(g) suppose at the temperature of a blast furnace the gibbs free energies of formation δgf of co2 and fe2o3 are −429./kjmol and −835./kjmol, respectively. calculate the minimum mass of coke needed to produce 6400.kg of pure iron. round your answer to 2 significant digits.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 23:30, johnnysteeler9934
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1

Chemistry, 23.06.2019 04:20, lelliott86
The lewis diagrams for magnesium and fluorine are shown below. what is the correct chemical formula for magnesium fluoride? a. mgf b. mg2f c. mgf2 d. mg2f3
Answers: 1

Chemistry, 23.06.2019 06:00, fjsdfj1284
Which change will decrease the number of effective collisions during a chemical reaction? a. adding a catalyst b. increasing the surface area c. decreasing the temperature d. increasing the reactant concentrations e. increasing the volume of the reactants
Answers: 2
Do you know the correct answer?
The reduction of iron(iii) oxide (fe2o3) to pure iron during the first step of steelmaking, 2fe2o3(s...
Questions in other subjects:


Mathematics, 10.07.2021 02:00


Mathematics, 10.07.2021 02:00


Social Studies, 10.07.2021 02:00





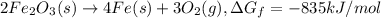 ...(1)
...(1)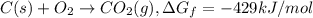 ...(2)
...(2)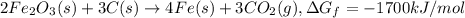 ...(3)
...(3)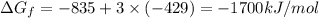
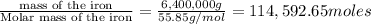
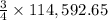 moles of coke.
moles of coke.