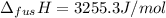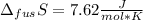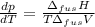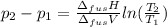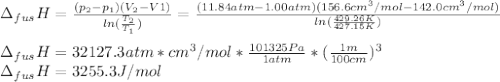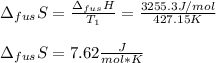The molar volume of a certain solid is 142.0 cm3 mol−1 at 1.00 atm and 427.15 k, its melting temperature. the molar volume of the liquid at this temperature and pressure is 152.6 cm3 mol−1. at 1.2 mpa the melting temperature changes to 429.26 k. calculate the enthalpy and entropy of fusion of the solid

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, WhiteWinterRose
What is the chemical formula of the following compound
Answers: 3


Do you know the correct answer?
The molar volume of a certain solid is 142.0 cm3 mol−1 at 1.00 atm and 427.15 k, its melting tempera...
Questions in other subjects:

Biology, 01.08.2019 10:30

Biology, 01.08.2019 10:30

Biology, 01.08.2019 10:30

Mathematics, 01.08.2019 10:30



History, 01.08.2019 10:30

Mathematics, 01.08.2019 10:30

History, 01.08.2019 10:30