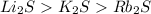Chemistry, 29.06.2019 16:30, dukkchild666
The melting points for the compounds li2s, rb2s, and k2s are 900°c, 530°c, and 840°c, respectively. list these three compounds in order of increasing lattice energy.

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 20:00, aksambo4707
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1
Do you know the correct answer?
The melting points for the compounds li2s, rb2s, and k2s are 900°c, 530°c, and 840°c, respectively....
Questions in other subjects:

Mathematics, 12.11.2020 19:50

English, 12.11.2020 19:50

English, 12.11.2020 19:50

Mathematics, 12.11.2020 19:50


Mathematics, 12.11.2020 19:50

Mathematics, 12.11.2020 19:50

Mathematics, 12.11.2020 19:50

Mathematics, 12.11.2020 19:50