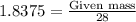The reaction of cr2o3 with silicon metal at high temperatures will make chromium metal. 2cro3(s) + 3si(> 4cr(l) + 3sio2 (s) the reaction is begun with 92.00 g of si and 112.00 g of cr2o3. how many grams of the excess reactant are left after the reaction is complete?

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 23.06.2019 10:30, dreamxette3119
Fill in the blanks for the following statements: the rms speed of the molecules in a sample of h2 gas at 300 k will be times larger than the rms speed of o2 molecules at the same temperature, and the ratio µrms (h2) / µrms (o2) with increasing temperature. a not enough information is given to answer this question b sixteen, will not change c four, will not change d four, will increase e sixteen, will decrease
Answers: 2
Do you know the correct answer?
The reaction of cr2o3 with silicon metal at high temperatures will make chromium metal. 2cro3(s) + 3...
Questions in other subjects:

History, 29.01.2020 05:43

Chemistry, 29.01.2020 05:43


Chemistry, 29.01.2020 05:43

Biology, 29.01.2020 05:43


English, 29.01.2020 05:43




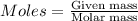 ....(1)
....(1)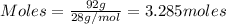
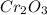
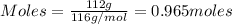
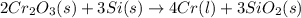
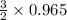 = 1.4475 moles of Silicon.
= 1.4475 moles of Silicon.