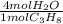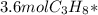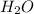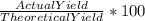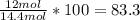Chemistry, 29.06.2019 20:30, jackrider1598
Phease asap given the following equation c3h8 + 5o2 = 3co2 +4h2o if i perform this reaction with 3.6 moles of c3h8 and an excess of oxygen gas , what is my theoretical yeild of water in moles? if i actually isolate 12 moles of waterwhat is my percent yeild?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:40, hannah2718
Asingle atom of an element has 21 neutrons, 20 electrons, and 20 protons. which element is it? ok o z
Answers: 1

Chemistry, 21.06.2019 23:00, SmolBeanPotato
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1

Chemistry, 21.06.2019 23:00, brapmaster764
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3

Chemistry, 22.06.2019 08:30, ebigham5117
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2
Do you know the correct answer?
Phease asap given the following equation c3h8 + 5o2 = 3co2 +4h2o if i perform this reaction with 3...
Questions in other subjects:

History, 20.04.2020 04:41

Mathematics, 20.04.2020 04:41





English, 20.04.2020 04:41

English, 20.04.2020 04:42

Mathematics, 20.04.2020 04:42

English, 20.04.2020 04:42


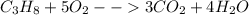
 =3.6 mol
=3.6 mol