
Chemistry, 29.06.2019 20:30, carafaith02
Choose the solvent below that would show the greatest freezing point lowering when used to make a 0.20 m nonelectrolyte solution. diethyl ether, kf = 1.79°c/m benzene, kf = 5.12°c/m chloroform, kf = 4.70°c/m ethanol, kf = 1.99°c/m carbon tetrachloride, kf = 29.9°c/m

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, Kjswagout5052
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2


Chemistry, 23.06.2019 15:30, tymiahill7244
Iv the concentration of ionic substances is important for the heart to beat. your heart responds to electrical impulses that travel through heart cells that are made up mostly of water. which properties of ionic compounds are important to support this function? solubility in water conductivity crystalline melting point الم" الا به done رلرلرللللہ و و او 8
Answers: 1
Do you know the correct answer?
Choose the solvent below that would show the greatest freezing point lowering when used to make a 0....
Questions in other subjects:



Social Studies, 23.12.2019 07:31


Mathematics, 23.12.2019 07:31

Mathematics, 23.12.2019 07:31

History, 23.12.2019 07:31


History, 23.12.2019 07:31

Mathematics, 23.12.2019 07:31


 will show the greatest freezing point lowering.
will show the greatest freezing point lowering.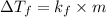
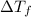 = lowering in freezing point
= lowering in freezing point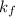 = molal depression constant
= molal depression constant



