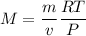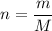Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, shadekashakay
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 09:00, phebusadrian01
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1


Chemistry, 22.06.2019 12:00, winterblanco
What is the lowest number energy level where a d sublevel is found
Answers: 1
Do you know the correct answer?
Question 12 hydrogen gas has a density of 0.090/gl , and at normal pressure and 2.57°c one mole of i...
Questions in other subjects:

Social Studies, 02.10.2021 23:00

History, 02.10.2021 23:00


History, 02.10.2021 23:00


Mathematics, 02.10.2021 23:00

History, 02.10.2021 23:00

Mathematics, 02.10.2021 23:00

Mathematics, 02.10.2021 23:00

Physics, 02.10.2021 23:00



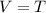


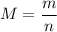 .... equation 1
.... equation 1
 .... equation 2
.... equation 2