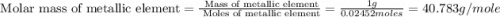1.00 g of a metallic element reacts completely with 300 cm3 of oxygen at 298 k and 1 atm pressure to form an oxide which contains o2– ions. the volume of one mole of gas at this temperature and pressure is 24.0 dm3. what could be the identity of the metal? a calcium b magnesium c potassium d sodium 11

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:50, deanlmartin
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1



Chemistry, 22.06.2019 04:30, salvadorperez26
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2
Do you know the correct answer?
1.00 g of a metallic element reacts completely with 300 cm3 of oxygen at 298 k and 1 atm pressure to...
Questions in other subjects:











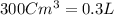
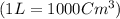
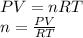
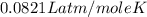
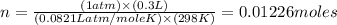
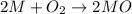
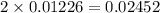 moles of metallic element
moles of metallic element