
Chemistry, 30.06.2019 02:30, brooke3493
Which statement is true? a) breaking a chemical bond releases energy. b) when energy is absorbed, the reaction is said to be endothermic and â†h is negative (–). c) bond dissociation energies are always positive numbers. d) the stronger the bond, the lower its bond dissociation energy. 2. based on the reaction, n2 + o2 → 2 no â†h = 43.2 kcal which statement is true? a) 43.2 kcal are consumed when 14.00 g of n2 reacts. b) 43.2 kcal are produced when 1.00 g of o2 reacts. c) 43.2 kcal are produced when 1.00 mole of o2 reacts. d) 43.2 kcal are consumed when 2.00 moles of no is produced.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, connienash95
Explain why scientists use shared characteristics to make cladograms.
Answers: 1

Chemistry, 22.06.2019 03:50, daniel9299
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3


Chemistry, 22.06.2019 18:30, sarahbug56
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2
Do you know the correct answer?
Which statement is true? a) breaking a chemical bond releases energy. b) when energy is absorbed, t...
Questions in other subjects:

English, 21.10.2020 04:01




Mathematics, 21.10.2020 04:01

Mathematics, 21.10.2020 04:01

Biology, 21.10.2020 04:01


Mathematics, 21.10.2020 04:01

History, 21.10.2020 04:01


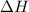 will be positive not negative.
will be positive not negative.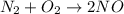
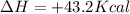
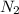 is reacted.
is reacted.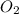 is reacted.
is reacted.



