Chemistry, 30.06.2019 07:00, khalaflaf2684
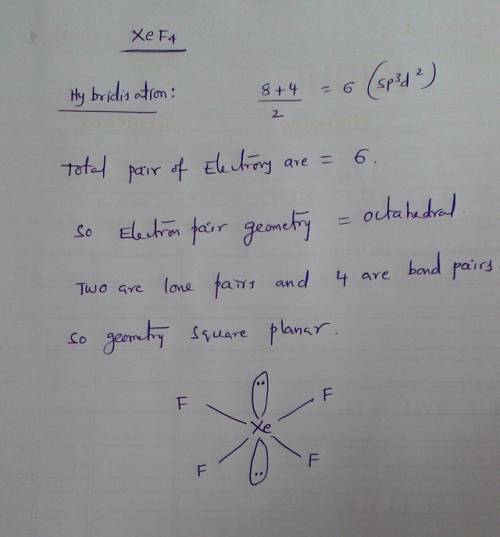
Give the electron geometry (eg), molecular geometry (mg), and hybridization for xef4. give the electron geometry (eg), molecular geometry (mg), and hybridization for xef4. eg=octahedral, mg=octahedral, sp3d2 eg=tetrahedral, mg=tetrahedral, sp3 eg=trigonal bipyramidal, mg=seesaw, sp3d eg=trigonal pyramidal, mg=trigonal pyramidal, sp3 eg=octahedral, mg=square planar, sp3d2

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, mrylenastewart
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 06:40, CylieTbh
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3


Chemistry, 23.06.2019 01:30, Nathaliasmiles
If a particle has z = 25 and 23 electrons, what is its charge?
Answers: 2
Do you know the correct answer?
Give the electron geometry (eg), molecular geometry (mg), and hybridization for xef4. give the elect...
Questions in other subjects:




Mathematics, 05.04.2021 19:10




Mathematics, 05.04.2021 19:10