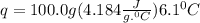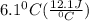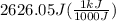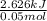A50.0 ml sample of a 1.00 m solution of cuso4 is mixed with 50.0 ml of 2.00 m koh in a calorimeter. cuso4 (1m) + 2koh (2m) cu(oh)2(s) + k2so4 (0.5m). the temperature of both solutions was 20.2 â°c before mixing and 26.3 â°c after mixing. the heat capacity of the calorimeter is 12.1 j/â°c. assume the specific heat and density of the solution after mixing are the same as those of pure water. from this data, calculate theî´h for the process if there is 0.05 mols of cuso4. (energy of the water + energy of the calorimeter)/(1000 x mol)= kj/mol of reaction

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, mayamabjishovrvq9
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 12:30, meghan2529
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 13:30, bryce99
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 13:30, citlalli30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2
Do you know the correct answer?
A50.0 ml sample of a 1.00 m solution of cuso4 is mixed with 50.0 ml of 2.00 m koh in a calorimeter....
Questions in other subjects:



Mathematics, 15.05.2021 04:30

Geography, 15.05.2021 04:30


Mathematics, 15.05.2021 04:30



Law, 15.05.2021 04:30

Mathematics, 15.05.2021 04:30


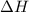 = -
= -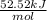
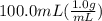
 = 26.3 - 20.2 = 6.1 degree C
= 26.3 - 20.2 = 6.1 degree C