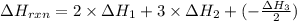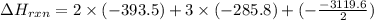Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:20, sindy35111
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l. s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3

Chemistry, 22.06.2019 20:50, iluminatioffial9699
One nanometer is equal to how many meters?
Answers: 2


Chemistry, 23.06.2019 01:00, birdman2540
Which of the following is in the lanthanide family? a) uranium b) promethium c) silver d) gold
Answers: 2
Do you know the correct answer?
C(graphite) + o2(g) → co2(g)δh o rxn = −393.5 kj/mol h2(g) + 1 2 o2(g) → h2o(l)δh o rxn = −285.8 kj/...
Questions in other subjects:



Mathematics, 11.12.2019 23:31



Mathematics, 11.12.2019 23:31

Chemistry, 11.12.2019 23:31


Biology, 11.12.2019 23:31



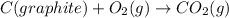
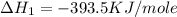
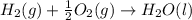
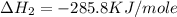
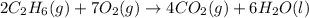
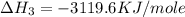
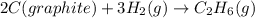
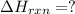
![2\times eq.(1)+\frac{1}{2}(eq.3)\text{[reversing equation 3 and dividing it by 2]}+3(eq.2)](/tpl/images/0034/7108/524c9.png)