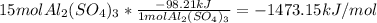Chemistry, 30.06.2019 19:00, lilchannelll4125
Al2(so4)3(s) + h2o(l) al2o3(s) + h2so4 (aq) calculate enthalpy formation for this reaction. balance the reaction. calculate the total enthalpy change that would occur from 15 moles of al2(so4)3

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, XxrazorxX11
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 16:00, yfnal3x
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 22.06.2019 17:00, calmicaela12s
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1

Chemistry, 23.06.2019 01:00, Alysssssssssssa
Which statement best describes isomers? a. isomers are alcohols that have the same functional group. b. isomers have at least one carbon-carbon double bond. c. isomers have the same molecular formula but different structural properties.
Answers: 1
Do you know the correct answer?
Al2(so4)3(s) + h2o(l) al2o3(s) + h2so4 (aq) calculate enthalpy formation for this reaction. balance...
Questions in other subjects:

Mathematics, 20.10.2020 20:01


Mathematics, 20.10.2020 20:01

Mathematics, 20.10.2020 20:01

Biology, 20.10.2020 20:01




Mathematics, 20.10.2020 20:01




![H_{reaction}^{0}=[H_{f}^{0}(Al_{2}O_{3}(s)) + (3*H_{f}^{0}(H_{2}SO_{4}(aq))] - [H_{f}^{0}(Al_{2}SO_{4}(aq)) + (3*H_{f}^{0}(H_{2}O(l))]](/tpl/images/0035/8926/80689.png)
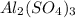 reacts will be=
reacts will be=