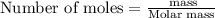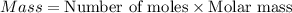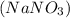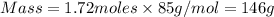Chemistry, 30.06.2019 22:00, kdencemartin
Select the correct answer. what is the mass of 1.72 moles of sodium nitrate? use the periodic table and the polyatomic ion resource. a. 85.0 g b. 91.2 g c. 146 g d. 273 g

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, smartboy2296
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 16:30, Eddie997
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Do you know the correct answer?
Select the correct answer. what is the mass of 1.72 moles of sodium nitrate? use the periodic table...
Questions in other subjects:

History, 14.11.2019 18:31

Biology, 14.11.2019 18:31



History, 14.11.2019 18:31





Chemistry, 14.11.2019 18:31