Chemistry, 01.07.2019 00:00, lalala1212
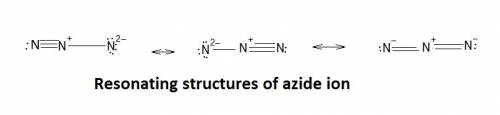
The azide ion, n−3, is a symmetrical ion, all of whose contributing structures have formal charges. draw three important contributing structures for this ion. draw the molecule by placing atoms on the grid and connecting them with bonds. include all nonbonding electrons. show the formal charges of all atoms.

Answers: 1
Similar questions


Chemistry, 13.07.2019 03:10, dreaaacx
Answers: 1

Chemistry, 02.10.2019 20:30, aj12381
Answers: 1
Do you know the correct answer?
The azide ion, n−3, is a symmetrical ion, all of whose contributing structures have formal charges....
Questions in other subjects:



Mathematics, 07.11.2019 17:31

English, 07.11.2019 17:31







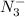 , is a symmetrical ion, all of whose contributing structures have formal charges.
, is a symmetrical ion, all of whose contributing structures have formal charges.