

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, tjjjjjjjjjjjjjjjjjjj
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 16:00, rorymartin04
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2

Chemistry, 22.06.2019 19:00, georgesarkes12
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2
Do you know the correct answer?
What is the numerical value of kc for the following reaction if the equilibrium mixture contains 0.5...
Questions in other subjects:



Geography, 19.09.2021 23:20



Mathematics, 19.09.2021 23:20

Mathematics, 19.09.2021 23:20

Mathematics, 19.09.2021 23:20

Mathematics, 19.09.2021 23:20


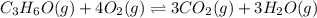
![Kc=\frac{[CO_2]^3[H_2O]^3}{[C_3H_6O][O_2]^4}](/tpl/images/0036/7459/52f34.png)
![Kc=\frac{[1.8]^3[2.0]^3}{[0.51][0.30]^4}](/tpl/images/0036/7459/c5aa6.png)




