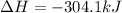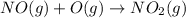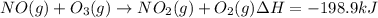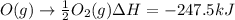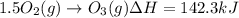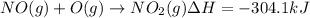Chemistry, 01.07.2019 00:30, jerseygirl1783
Calculate the enthalpy change for the reaction no(g) + o(g) → no2(g) from the following data: no(g) + o3(g) → no2(g) + o2(g) δh = –198.9 kj o3(g) → 1.5o2(g) δh = –142.3 kj o2(g) → 2o(g) δh = 495.0 kj

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, zamirareece17
1. list three scientific reasons cockroaches may fly.
Answers: 1

Chemistry, 22.06.2019 07:30, 10040813
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 17:00, emma3216
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1
Do you know the correct answer?
Calculate the enthalpy change for the reaction no(g) + o(g) → no2(g) from the following data: no(g)...
Questions in other subjects:



Mathematics, 18.10.2019 17:30

Spanish, 18.10.2019 17:30

Mathematics, 18.10.2019 17:30


Biology, 18.10.2019 17:30

History, 18.10.2019 17:30

History, 18.10.2019 17:30

Geography, 18.10.2019 17:30