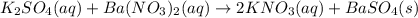Chemistry, 01.07.2019 04:30, chelsearodi3985
Use the solubility rules and periodic table to predict the product that will precipitate out in the reaction


Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, kkmonsterhigh18
The diagram below shows a cell placed in a solution. a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution. only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it. it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2

Chemistry, 22.06.2019 17:00, abbygailgo674
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1

Chemistry, 22.06.2019 20:30, huangjianhe135
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3
Do you know the correct answer?
Use the solubility rules and periodic table to predict the product that will precipitate out in the...
Questions in other subjects:

Mathematics, 10.06.2021 20:50

Mathematics, 10.06.2021 20:50


Chemistry, 10.06.2021 20:50


English, 10.06.2021 20:50


Arts, 10.06.2021 20:50

Biology, 10.06.2021 20:50

Mathematics, 10.06.2021 20:50



 and
and  and both of these are soluble. The double displacement reaction takes place in which the ion exchange takes place. sulfate ion goes to barium and nitrate ion goes to potassium and the products are
and both of these are soluble. The double displacement reaction takes place in which the ion exchange takes place. sulfate ion goes to barium and nitrate ion goes to potassium and the products are  and
and