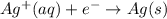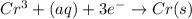Chemistry, 01.07.2019 06:30, trosclairozlynn02
The following equations are half reactions and reduction potentials. ag+ (aq) + e– ag(s) has a reduction potential of +0.80 v. cr3+ (aq) + 3e– cr(s) has a reduction potential of –0.74 v. which statement best compares the substances in these half reactions? a silver ion gains electrons more easily and is a stronger oxidizing agent than a chromium(iii) ion. a silver ion loses electrons more easily and is a stronger oxidizing agent than a chromium(iii) ion. a silver ion gains electrons more easily and is a stronger reducing agent than a chromium(iii) ion. a silver ion loses electrons more easily and is a stronger reducing agent than a chromium(iii) ion. the answer is a

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:40, marknjenbennetp3j1v1
Listen base your answer to the question on the information below. propane is a fuel that is sold in rigid, pressurized cylinders. most of the propane in a cylinder is liquid, with gas in the space above the liquid level. when propane is released from the cylinder, the propane leaves the cylinder as a gas. propane gas is used as a fuel by mixing it with oxygen in the air and igniting the mixture, as represented by the balanced equation below. c3h8(g) + 5o2(g) → 3co2(g) + 4h2o() + 2219.2 kja small amount of methanethiol, which has a distinct odor, is added to the propane to consumers detect a propane leak. in methanethiol, the odor is caused by the thiol functional group (–sh). methanethiol, ch3sh, has a structure that is very similar to the structure of methanol. what is the correct structural formula for a molecule of methanethiol
Answers: 3


Chemistry, 22.06.2019 16:30, ccispoppin12
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 23.06.2019 01:50, UncleVictor5188
Ablock of aluminum is dropped into a graduated cylinder with an initial volume of water at 75ml and the volumes rises to 90ml. if the block has a mass of 40.5 g what is its density ?
Answers: 1
Do you know the correct answer?
The following equations are half reactions and reduction potentials. ag+ (aq) + e– ag(s) has a reduc...
Questions in other subjects:

Social Studies, 21.01.2021 20:40


Mathematics, 21.01.2021 20:40


Biology, 21.01.2021 20:40


Chemistry, 21.01.2021 20:40

Mathematics, 21.01.2021 20:40

History, 21.01.2021 20:40

Biology, 21.01.2021 20:40