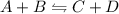Which of the following is not a result when a change to an equilibrium system is applied? increasing the rate of the forward reaction will cause a shift to the left. increasing the rate of the reverse reaction will cause a shift to the left. decreasing the rate of the forward reaction will cause a shift to the left. decreasing the rate of the reverse reaction will cause a shift to the right.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, chefdnguyen
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1

Chemistry, 22.06.2019 11:00, justarando
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 12:00, KKHeffner02
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 15:20, munziruddin204
Which description best characterizes the motion of particles in a solid?
Answers: 2
Do you know the correct answer?
Which of the following is not a result when a change to an equilibrium system is applied? increasin...
Questions in other subjects:


Computers and Technology, 12.08.2019 23:30