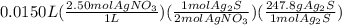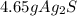Chemistry, 01.07.2019 21:30, jadenpmoore2008
Select the correct answer. if a silver nitrate solution is added to excess sodium sulfide, this reaction takes place: 2agno3(aq) + na2s(aq) → ag2s(s) + 2nano3(aq). suppose you use 0.0150 liter of a 2.50 m solution of silver nitrate. assuming the reaction goes to completion, how much silver sulfide is produced? use the periodic table. a. 1.49 g b. 4.65 g c. 9.30 g d. 18.6 g

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:10, cameronbeaugh
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Do you know the correct answer?
Select the correct answer. if a silver nitrate solution is added to excess sodium sulfide, this reac...
Questions in other subjects:


History, 20.09.2020 15:01




Mathematics, 20.09.2020 15:01

Mathematics, 20.09.2020 15:01




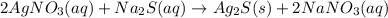
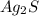 = 2(107.87)+32.06 = 247.8 g per mol
= 2(107.87)+32.06 = 247.8 g per mol