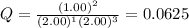Chemistry, 02.07.2019 05:00, lucystudies
What is the reaction quotient, q, for this system when [n2] = 2.00 m, [h2] = 2.00 m, and [nh3] = 1.00 m at 472°c?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:10, purplefish53
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 16:40, westball101
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3
Do you know the correct answer?
What is the reaction quotient, q, for this system when [n2] = 2.00 m, [h2] = 2.00 m, and [nh3] = 1.0...
Questions in other subjects:


Social Studies, 07.12.2021 01:50

Mathematics, 07.12.2021 01:50


Biology, 07.12.2021 01:50


History, 07.12.2021 01:50


Social Studies, 07.12.2021 01:50

Advanced Placement (AP), 07.12.2021 01:50


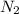 = 2.00 M
= 2.00 M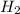 = 2.00 M
= 2.00 M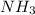 = 1.00 M
= 1.00 M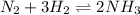
![Q=\frac{[Product]^p}{[Reactant]^r}\\Q=\frac{[NH_3]^2}{[N_2]^1[H_2]^3}](/tpl/images/0041/3273/399e0.png)