
Type the correct answer in the box. express the answer to three significant figures. given: ch4 + 2o2 → co2 + 2h2o, δh = -890 kj/mol how much energy is released when 59.7 grams of methane (ch4) reacts with oxygen? the combustion of 59.7 grams of methane releases 34.5 kilojoules of energy.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, murtaghliam1
Word equation for k(s) +h2o(l) yield koh (aq) + h2
Answers: 3

Chemistry, 22.06.2019 23:30, sanociahnoel
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1

Chemistry, 22.06.2019 23:30, ninilizovtskt
If maltose undergoes hydrolysis what subunits does it results to?
Answers: 2

Chemistry, 23.06.2019 01:00, birdman2540
Which of the following is in the lanthanide family? a) uranium b) promethium c) silver d) gold
Answers: 2
Do you know the correct answer?
Type the correct answer in the box. express the answer to three significant figures. given: ch4 + 2...
Questions in other subjects:



History, 12.11.2020 16:50





English, 12.11.2020 16:50

Mathematics, 12.11.2020 16:50


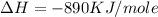
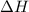 is in negative that means the energy is releasing.
is in negative that means the energy is releasing.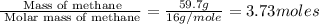
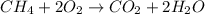
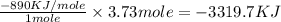 of energy
of energy



