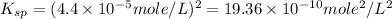Chemistry, 02.07.2019 09:30, jjortiz3137
The solubility of baco3(s) in water at a certain temperature is 4.4 10–5 mol/l. calculate the value of ksp for baco3(s) at this temperature.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:10, lilque6112
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 22.06.2019 05:20, jtingley0502
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1
Do you know the correct answer?
The solubility of baco3(s) in water at a certain temperature is 4.4 10–5 mol/l. calculate the value...
Questions in other subjects:

History, 28.10.2020 20:00

Mathematics, 28.10.2020 20:00

Mathematics, 28.10.2020 20:00




Chemistry, 28.10.2020 20:00


History, 28.10.2020 20:00


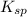 for
for 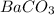 is
is 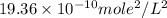 .
.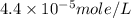
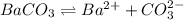
![K_{sp}=[Ba^{2+}][CO^{2-}_3]](/tpl/images/0042/0615/8f6e9.png)
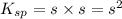
![[Ba^{2+}]=[CO^{2-}_3]=s=4.4\times 10^{-5}mole/L](/tpl/images/0042/0615/c600c.png)