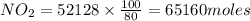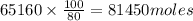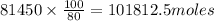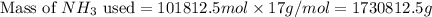Chemistry, 02.07.2019 09:30, hehefjf8610
What mass of nh3 in grams must be used to produce 1.81 tons of hno3 by the ostwald process, assuming an 80.0 percent yield in each step (1 ton=2000 lb; 1 lb= 453.6 g)?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, meghan2529
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 23:00, jolainjoseph01998
What element has similar physical and chemical properties as boron.
Answers: 1


Do you know the correct answer?
What mass of nh3 in grams must be used to produce 1.81 tons of hno3 by the ostwald process, assuming...
Questions in other subjects:

English, 18.12.2020 22:30

Mathematics, 18.12.2020 22:30



Geography, 18.12.2020 22:30



History, 18.12.2020 22:30


Mathematics, 18.12.2020 22:30


 used is 1730812.5 grams.
used is 1730812.5 grams.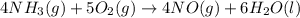
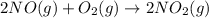
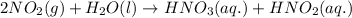
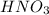 produced = 1.81 tons
produced = 1.81 tons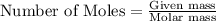 ....(1)
....(1)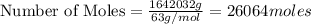
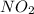 , theorectically (From Step-III) = 2 × 26064 = 52128 moles
, theorectically (From Step-III) = 2 × 26064 = 52128 moles