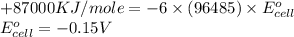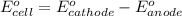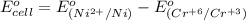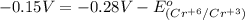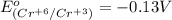3ni2+(aq) + 2 cr(oh)3(s) + 10 oh− (aq) → 3 ni(s) + 2 cro42−(aq) + 8 h2o(l) δg∘ = +87 kj/mol given the standard reduction potential of the half-reaction ni2+(aq) + 2 e− → ni(s) e∘red = -0.28 v, calculate the standard reduction potential of the half-reaction cro42−(aq) + 4 h2o(l) + 3 e− → cr(oh)3(s) + 5 oh−(aq)

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:00, aksambo4707
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1

Chemistry, 22.06.2019 22:00, notearslefttocry14
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2
Do you know the correct answer?
3ni2+(aq) + 2 cr(oh)3(s) + 10 oh− (aq) → 3 ni(s) + 2 cro42−(aq) + 8 h2o(l) δg∘ = +87 kj/mol given th...
Questions in other subjects:

Mathematics, 09.11.2020 23:00


Engineering, 09.11.2020 23:00








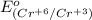 is -0.13 V.
is -0.13 V.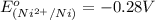
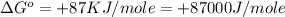 (1 KJ = 1000 J)
(1 KJ = 1000 J)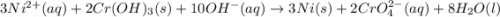
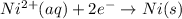
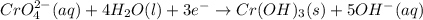
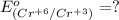
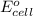 by using formula,
by using formula,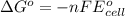
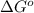 = Gibbs's free energy
= Gibbs's free energy