
Chemistry, 02.07.2019 16:00, ianmorales4894
What is the maximum number of electrons in an atom that can have the following quantum numbers? a) n = 5, l = 3, ml = +3 b) n = 3, l = 1, ml = -1

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:10, browndalton55
Which equation represents a fission reaction? o "9n+h—150 o 235u + n—190cs + rb+25 o be + he—1c + in o 28 np —> 2390 pute
Answers: 1

Chemistry, 21.06.2019 18:30, Tyrant4life
Amass of 100.0 g of solute is dissolved in water so that 850. ml of a 0.7500 m solution has been prepared. what is the molar mass of the solute?
Answers: 2

Chemistry, 22.06.2019 12:30, kaliyab191
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 14:30, villarrealc1987
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2
Do you know the correct answer?
What is the maximum number of electrons in an atom that can have the following quantum numbers? a)...
Questions in other subjects:

Mathematics, 04.03.2021 02:50


Mathematics, 04.03.2021 02:50


Mathematics, 04.03.2021 02:50


Mathematics, 04.03.2021 02:50

Mathematics, 04.03.2021 02:50

Mathematics, 04.03.2021 02:50

Mathematics, 04.03.2021 02:50


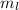 = +3 is 2 (two).
= +3 is 2 (two).



