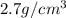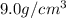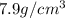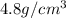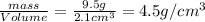Chemistry, 02.07.2019 23:30, nehemiahj85
Marcus measured the masses and volumes of samples of four different substances, and he calculated their densities. the table shows marcus’s measured and calculated values. substance mass (g) volume (cm3) density (g/cm3) aluminum 5.7 2.1 2.7 copper 14.4 1.6 9.0 iron 9.5 1.2 7.9 titanium 8.6 1.8 4.8 next, marcus obtained another sample, which is made of one of the four substances that he had already measured. the table shows marcus’s measured values for this unknown sample. substance mass (g) volume (cm3) ? 9.5 2.1 what is the unknown sample made of?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, sannai0415
Zinc + lead(ii) nitrate yield zinc nitrate + leadwhat's the chemical equation for this?
Answers: 1



Chemistry, 22.06.2019 19:00, HaydenSturgis1
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3
Do you know the correct answer?
Marcus measured the masses and volumes of samples of four different substances, and he calculated th...
Questions in other subjects:

Mathematics, 29.10.2020 01:20

History, 29.10.2020 01:20

English, 29.10.2020 01:20

Social Studies, 29.10.2020 01:20


Mathematics, 29.10.2020 01:20




Physics, 29.10.2020 01:20