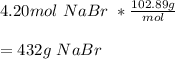Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, Sarahinator04
0.09 moles of sodium sulfate in 12 ml of solution
Answers: 3


Chemistry, 22.06.2019 18:30, chinadoll24
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2
Do you know the correct answer?
James needs 4.20 moles of nabr for an experiment. how many grams of nabr must he measure out to have...
Questions in other subjects:

English, 14.11.2019 04:31



Biology, 14.11.2019 04:31



Mathematics, 14.11.2019 04:31


Mathematics, 14.11.2019 04:31

Mathematics, 14.11.2019 04:31