
Chemistry, 03.07.2019 04:00, guzmangisselle
Why are saturated hydrocarbons generally less reactive than unsaturated hydrocarbons? a. atoms are not easily added to molecules with single bonds. b. atoms are not easily added to molecules with double bonds. c. saturated hydrocarbons have fewer carbon atoms than unsaturated hydrocarbons.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, cheyennecarrillo14
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 14:00, jivsf
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3

Chemistry, 22.06.2019 14:30, neidaq12345
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2
Do you know the correct answer?
Why are saturated hydrocarbons generally less reactive than unsaturated hydrocarbons? a. atoms are...
Questions in other subjects:

Mathematics, 27.06.2019 22:30

Social Studies, 27.06.2019 22:30

Social Studies, 27.06.2019 22:30





Mathematics, 27.06.2019 22:30

Social Studies, 27.06.2019 22:30

Mathematics, 27.06.2019 22:30


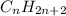
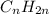 and
and 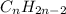
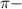 bonds. They are weaker than
bonds. They are weaker than 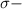 bonds and thus can be easily broken. Addition reactions is more favorable for the compounds having
bonds and thus can be easily broken. Addition reactions is more favorable for the compounds having 



