Chemistry, 03.07.2019 10:30, kaliahgrey
Use the balanced equation to work the following problem: cacl2 + 2agno3 → 2 agcl + ca(no3)2 how many grams of agcl (molar mass=143 g/mol) is formed when 1.00 gram of agno3 (molar mass=170 g/mol) reacts? 1.00 g 0.00588 g 1.19 g 0.841 g

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, lpssprinklezlps
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 12:30, MrSavannahCat
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1

Chemistry, 22.06.2019 14:30, davidrodriguez122001
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 19:20, halledoll2002
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3
Do you know the correct answer?
Use the balanced equation to work the following problem: cacl2 + 2agno3 → 2 agcl + ca(no3)2 how man...
Questions in other subjects:


Mathematics, 23.10.2021 14:00


Mathematics, 23.10.2021 14:00


Mathematics, 23.10.2021 14:00





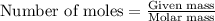
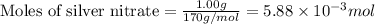
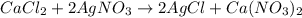
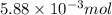 of silver nitrate will produce =
of silver nitrate will produce = 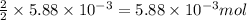 of AgCl
of AgCl