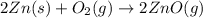Chemistry, 03.07.2019 11:30, vlactawhalm29
When zinc metal reacts with oxygen gas, 2zn(s) + o2(g) → 2zno(g), large amounts of light and heat are released. a student states that this reaction is a combustion reaction but not a redox reaction. do you agree? defend your answer by explaining whether or not it meets the requirements of each type of reaction. asap

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:50, britotellerialuis
Evaluate this exponential expression,8. (2 + 3)2 – 42
Answers: 3

Chemistry, 22.06.2019 18:10, sangamlama
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Do you know the correct answer?
When zinc metal reacts with oxygen gas, 2zn(s) + o2(g) → 2zno(g), large amounts of light and heat ar...
Questions in other subjects:

English, 21.05.2020 07:00






Mathematics, 21.05.2020 07:00

English, 21.05.2020 07:00

Health, 21.05.2020 07:00

Mathematics, 21.05.2020 07:00