
Chemistry, 03.07.2019 11:30, lobatospitones
In this reaction, how does the rate of forward reaction vary with the concentration of the product? 2h2s(g) ⇌ 2h2(g) + s2(g) it increases with an increase in the concentration of s2(g). it decreases with a decrease in the concentration of h2(g). it increases with a decrease in the concentration of h2(g). it decreases with an increase in the concentration of s2(g). it decreases with increase in the concentration of h2(g). multiple answers

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, itzyagirlshy
Determine the number o moles of ions/atoms/particle in the following: 2.50 miles of k2s (let me know how to do)
Answers: 1

Chemistry, 22.06.2019 03:10, emilyplays474
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2

Chemistry, 22.06.2019 12:30, hala201490
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Do you know the correct answer?
In this reaction, how does the rate of forward reaction vary with the concentration of the product?...
Questions in other subjects:

Mathematics, 10.10.2021 05:00

History, 10.10.2021 05:00



Chemistry, 10.10.2021 05:00


Physics, 10.10.2021 05:00


Mathematics, 10.10.2021 05:00


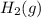 . D) It decreases with increase in the concentration of
. D) It decreases with increase in the concentration of 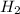 or
or 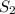 is increased, the reaction will shift in backward direction and the rate of forward direction decreases.
is increased, the reaction will shift in backward direction and the rate of forward direction decreases.



